Sequence Comparison of Gonadotropin Subunits in Laboratory Animals
In this page, we show you our study in which we determined amino acid sequences for gonadotropin subunits in laboratory animals in order to determine the molecular basis for an efficient superovulation technique applicable to a wide range of animal species.
Any questions, any suggestions, and any improvements are always welcome. Please contact us if you have any of them.
Introduction
The induction of superovulation by gonadotropin treatment is a basic technique for manipulating the embryos of laboratory animals. To understand the molecular basis of this technique, we examined and compared the cDNA sequences of the pituitary gonadotropin subunits and their receptors in several animals, and found that homology between exogenous and endogenous gonadotropins is a key factor in efficient superovulation. Therefore, we wished to collect sequence information on the gonadotropins and their receptors for animals in which embryo manipulation techniques are currently applied or will be applied in the future.
Classification of animals used in this study
We determined gonadotropin subunit sequences of a total of ten laboratory animals: seven sciurognath rodents, two hystricognath rodents, and a laboratory rabbit as shown below, and compared them with those of other mammals.
| Order | suborder | family | subfamily | species | breed |
|---|---|---|---|---|---|
| Rodentia | Sciurognathi | Sciuromorpha | (not used in this study) | ||
| Myomorpha | Arvicolinae | Japanese grass vole | |||
| Cricetinae | Syrian hamster | ||||
| Armenian hamster | |||||
| Chinese hamster | |||||
| Djungarian hamster | |||||
| Gerbillinae | Mongolian gerbil | ||||
| Murinae | Mouse | ||||
| Rat | |||||
| Mastomys | |||||
| Hystricognathi | Caviidae | Guinea pig | |||
| Octodontidae | Degu | ||||
| Lagomorpha | Leporidae | Rabbit | JW (Japanese White) | ||
| NZW (New Zealand White) | |||||
| Dutch |
Syrian hamsters are also called Goldn hamsters.
Reproductive features of laboratory animals
To establish and improve superovulation induction methods in a wide variety of laboratory animals, we should consider the species-specific endocrinological and reproductive processes of target animals. This table illustrates reproductive features of various laboratory animals. Note that various reproductive systems work even in a single order of mammals (Rodentia).
| Mouse/Rat/Mastomys | Hamsters | Vole | Guinea pig | Rabbit | |
|---|---|---|---|---|---|
| Estrous cycle | No luteal phase (4-5 days) | No luteal phase (~4 days) | Persistent estrus ? | Both folliclar and lueal phases (~20 days) | Persistent estrus |
| Ovulation | Spontaneous | Spontaneous | Copulatory | Spontaneous | Copulatory |
| Activation of CL | Copulation | Copulation | (Copulation ?) | Spontaneous | (Copulation ?) |
| Litter size | Large | Large | Intermediate | Small | Large |
| Pregnancy period | Short (~20 days) | Short (15-21 days) | Short (22-25 days) | Long (~65 days) | Intermediate (~1 month) |
| New born offspring | No hair coat, closed eyes | No hair coat, closed eyes | No hair coat, closed eyes | Hair coat, open eyes | No hair coat, closed eyes |
| Misc. | Vaginal closure membrane |
Gonadotropins
Gonadotropins are hormones secreted from pituitary and placenta. Gonadotrpins include follicle stimulating hormone (FSH), luteinizing hormone (LH) and chorionic gonadotropins(CG) which are only produced in primates and horses. These horomones are heterodimers consisting of α subunits (Common-α, aka, CGα and α-GSU), which are common to three pituitary glycoprotein hormones (FSH, LH and TSH), and hormone-specific β subunits (FSHβ, LHβ, and CGβ). In this study, we used pitutaries only because no laboratory animal used in this study produce chorionic gonadotropins.
Common-α and LHβ cDNAs are approxymately 600 nt long (except polyA tails), whereas FSHβ cDNA has long 3'-UTR; and is approximately 1,500-2,000 nt long depending on species. Length of amino acid sequences of common-α, FSHβ and LHβ including signal peptides are 120, 130, and 143 aa long, respectively (See Boucefield et al., 1994 for details).
Comparison of amino acid sequences of gonadotropin subunits
Total RNA was extracted from pituitary glands using the RNeasy Mini Kit (Qiagen). The full cDNA sequences of common-&aplha;, FSHβ and LHβ subunit proteins were determined by a combination of both 5'- and 3'-rapid amplification of cDNA ends (RACE) with the SMART RACE cDNA amplification kit (Clontech), followed by direct sequencing the RACE products. Full sequences were obtained by combining two overlapping sequences of the 5'- and 3'-RACE products. Gene specific primers for RACE were designed on a basis of rat sequences.
A dot indicates an amino acid residue that is identical at the corresponding position in the mouse sequence of each subunit (top row). Dashes indicate sequence gaps inserted by the CLUSTAL W software.
Amino acid sequences of Common-α (α-GSU, CGα)
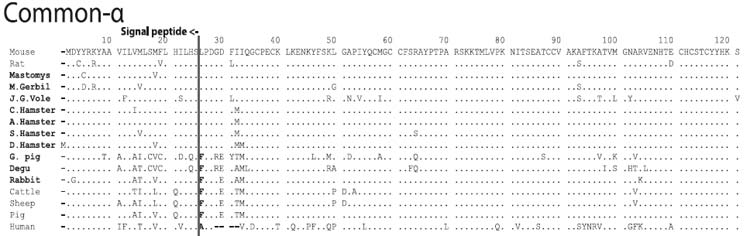
Note that sciurognath rodents have a leucine at the N-terminus of mature common-α, whereas most other mammals, including hystricognath rodents, have a phenylalanine; the signal sequence and the N-terminal region of mature sequence of human common-α are quite different from those of other mammals.
Amino acid sequences of FSHβ
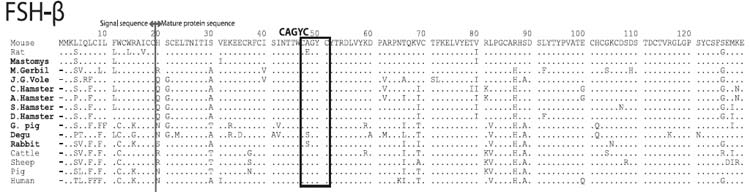
FSH-β subunits start with MM in the subfamily Murinae (mouse, rat, and mastomys), whereas those of other mammals start with M.
Amino acid sequences of LHβ

The CAGYC sequences (responsible for binding to α-subunit) are highly conserved among mammals; Two loop regions (responsible for receptor binding) suggest that LH-βs are roughly divided into three groups: rodent-type, artiodactyl-type, and primate-type.
Molecular phylogeny
Neighbor-joining trees for both subunits created by MEGA3.1 software (Kumar et al, 2004) indicate that the hystricognath rodents are evolutionarily distant from sciurognath rodents and that the rabbit stands at an intermediate position between rodents and domestic animals.
Common-α (α-GSU, CGα)
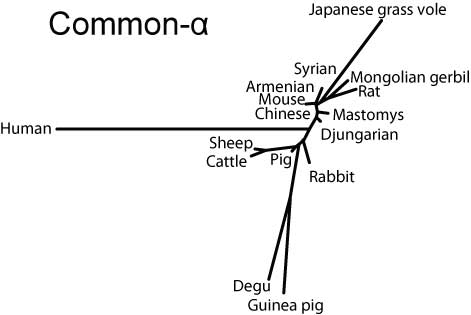 NJ-tree of Common-α subunits (SVG version)
NJ-tree of Common-α subunits (SVG version)
FSH-β
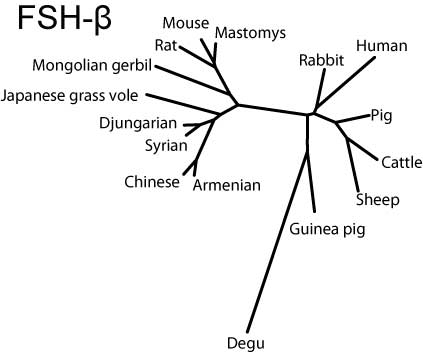 NJ-tree of FSH-β subunits (SVG version)
NJ-tree of FSH-β subunits (SVG version)
LH-β
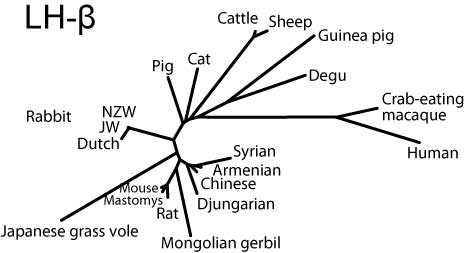 NJ-tree of LH-β subunits (SVG version)
NJ-tree of LH-β subunits (SVG version)
Summary
The alignment of the deduced amino acid sequences indicates an overall similarity of gonadotropin subunits among mammalian species. This high homology might explain the eCG, the gonadotropin from the horse origin, can act as a potent inducer of ovarian follicle growth in laboratory animals. Human common-α has some deletions at the N-teriminus of the mature protein, suggesting that gonadotropins from the human origin may have lower affinities to gondotropin receptors of the other mammals. If it is a case, we shoud choose gonadotropins from mammalian origins except humans as superovulation inducers. On the other hand, gonadotropins of hystricognathi animals are more homologus to human and artilodactyl animals than to other rodents, suggesting that the human preparation of gonadotropin would be effective.
Guinea pig FSH-receptor sequence (AY082514) is highly homologus to human counterpart. We confirmed that repetitive administration of the human FSH preparations (e.g., human menopausal gonadotropins) induced superovulation in the guinea pig (Suzuki, et al., 2003).
List of Accession Numbers
List of Accession Numbers (determined in the present study)
| Species | Common-α | FSHβ | LHβ |
|---|---|---|---|
| Mongolian gerbil | AF303351 | AY376457 | AY369077 |
| Mastomys | AF307149 | AY458603 | AY353073 |
| Japanese grass vole | AF307150 | AB262180 | AB262179 |
| Syrian hamster | AF307148 | AB241062 | AY353074 |
| Armenian hamster | AB235912 | AB235911 | AB233028 |
| Chinese hamster | AB248597 | AB248599 | AB248598 |
| Djungarian hamster | AB250761 | AB252645 | AB250762 |
| Guinea pig | AF257213 | AF257212 | AY373317 |
| Degu | AB262181 | AB262182 | AB262183 |
| Rabbit | AF318299 (JW) | AY614704 (JW) | AY614703 (JW) AB235913 (NZW) AB235914 (Dutch) |
List of Accession Numbers (retrieved from databases)
| Species | Common-α | FSHβ | LHβ |
|---|---|---|---|
| Mouse | J00643 | NP_032071 | NM_008497 |
| Rat | V01252 | P18427 | J00749 |
| Pig | D00767 & D00768 | P01228 | NM_214080 |
| Sheep | X16977 | P01227 | NM_001009380 |
| Cattle | X00050 | NP_776485 | NM_173930 |
| Human | NM000735 | P01225 | NM_000894 |
| Cat | - | - | NM_001009277 |
| Crab-eating macaque | - | - | AJ781396 |
Acknowledgements
We thank Dr. K. Tsuchiya of Miyazaki Medical College (at the time of provision) for degu pituitaries, Dr. K. Ike of Nippon Veterinary and Life Science University for Djuangarian hamster pituitaries, and Dr. S. Kamijo of Mitsubishi Kagaku Institute of Life Sciences for Chinese hamster pituitaries.
References
Review
- Bousfield, G. R., Perry, W. M., Ward, D. N., (1994) Gonadotropins. In Knobil, E., Neill, J. D. (eds.), The physiology of reproduction. 2nd Edition, Raven Press, Ltd., New York, 1749-1792.
Rabbit LHβ sequence and its pyroglutamylation
- Glenn, S. D., Nahm, H. S., Ward, D. N. (1984) The amino acid sequence of the rabbit lutropin beta subunit. J. Protein Chem. 3:259-273.
Software for production of molecular phylogeny
- Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated Software for Molecular Evolutionary Genetics Analysis and Sequence Alignment Briefings in Bioinformatics 5:150-163.
Our papers related to this study
- Suzuki, O. (2006) Sequence comparison of gonadotropin subunits in laboratory animals for improvement of superovulation techniques. Kansai J. Lab. Anim. 27:72-75. [in Japanese] (This page is mainly based on this paper)
- Koura, M., Handa, H., Noguchi, Y., Takano, K., Yamamoto, Y., Matsuda, J., Suzuki, O. (2004) Sequence analysis of cDNA encoding follicle-stimulating hormone and luteinizing hormone β-subunits in the Mongolian gerbil (Meriones unguiculatus). Gen. Comp. Endocrinol. 136:406-410.
- Noguchi, Y., Takano, K., Koura, M., Uchio-Yamada, K., Matsuda, J., Suzuki, O. (2006) Sequence analysis of cDNA encoding rabbit follicle-stimulating hormone β-subunit precursor protein. Gen. Comp. Endocrinol. 147:231-235.
- Suzuki, O., Mochida, K., Yamamoto, Y., Noguchi, Y., Takano, K., Matsuda, J., Ogura, A. (2002) Comparison of glycoprotein hormone α-subunits of laboratory animals. Mol. Reprod. Dev. 62:335-342.
- Suzuki, O., Koura, M., Noguchi, Y., Takano, K., Yamamoto, Y., Matsuda, J. (2003) Optimization of superovulation induction by human menopausal gonadotropin in guinea pigs based on follicular waves and FSH-receptor homologies. Mol. Reprod. Dev. 64:219-225.
- Suzuki, O., Koura, M., Noguchi, Y., Takano, K., Uchio-Yamada, K., Matsuda, J. (2007) Analyses of the cDNA and genomic DNA sequences encoding the luteinizing hormone β-subunit precursor protein in the rabbit. Gen. Comp. Endocrinol. 150:514-519.
- Takano, K., Koura, M., Noguchi, Y., Yamamoto, Y., Uchio-Yamada, K., Matsuda, J., Suzuki, O. (2004) Sequence analysis of cDNA encoding follicle-stimulating hormone and luteinizing hormone β-subunits in the Mastomys (Praomys coucha). Gen. Comp. Endocrinol. 138:281-286.
